House Democrats push FDA to lift safety protocols for abortion drugs
Pro-abortion legislators in the U.S. House of Representatives are calling on the Food and Drug Administration to permanently discard safety protocols for dispensing drugs that induce a miscarriage to terminate an early first-trimester pregnancy, commonly referred to as the “Risk, Evaluation and Mitigation Strategies.”
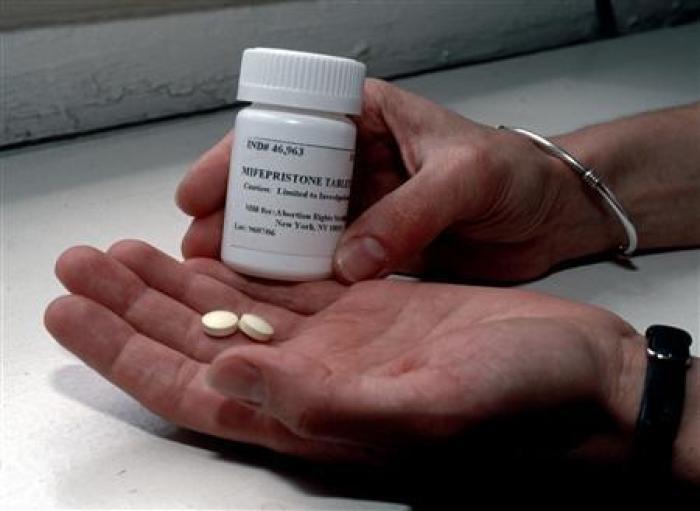
A group of more than six dozen House Democrats, led by Rep. Carolyn Maloney, D-N.Y., introduced a resolution Thursday that would express “the sense of the House of Representatives that policies governing abortion care should be equitable and based on science.” The resolution specifically focused on the abortion pills, also referred to as a chemical abortion or medication abortion. A chemical abortion has two components: mifepristone and misoprostol.
The lawmakers claim that the REMS have “no medical basis” and echoed the FDA’s analysis that abortion pills’ “efficacy and safety have become well-established both by research and experience, and serious complications have proven to be extremely rare.” Rep. Diana DeGette, D-Colo., a co-sponsor of the resolution, elaborated on their goal in a statement.
“As chair of the Pro-Choice Caucus, we are committed to eliminating all unnecessary barriers to abortion care, including the onerous and unnecessary restrictions on the use of medication abortion,” she said. “We look forward to working with the FDA to ensure any policies regarding the use of mifepristone are based solely on science and evidence, not policy or ideology.”
The resolution comes after the FDA approved the temporary suspension of the REMS earlier this year, citing the COVID-19 pandemic as the reason for such a decision. Pro-abortion politicians and activists believe women should have the ability to obtain abortion-inducing drugs in the mail because of the dangers posed by the pandemic. The pro-abortion lawmakers who co-sponsored the resolution indicated their desire to make these changes to the REMS permanent.
As explained in the resolution, the REMS “require that it be dispensed to patients in person, that healthcare providers who prescribe mifepristone receive certification before doing so, and that certified prescribers obtain a signed safety agreement from patients before prescribing mifepristone to them.”
The lawmakers' resolution noted that “on May 7, 2021, the FDA indicated that it is conducting an evidence-based review of the REMS for mifepristone,” the lawmakers argued that policies surrounding the distribution of the abortion pill should “be grounded in science and based on a scientific review of available medical evidence” and “ensure equitable access for patients harmed by restrictions ..."
This is not the first time the FDA has been pushed to change its safety guidelines for the use of abortion-inducing drugs.
When mifepristone first came out, the FDA had a protocol for it to be used through seven weeks of pregnancy, or 49 days from conception, according to Sue Turner, director of Physicians for Life.
Because many abortion clinics in the U.S. were ignoring the FDA’s protocol and using the drug in chemical abortions up to 60 days, states began passing regulations saying they had to follow the FDA’s protocol. “They didn’t want to have to follow the FDA protocol, so [then President] Obama made the FDA change it to the later date, the 60 days, to match up with what the abortion providers were doing,” Turner told The Christian Post in a previous interview.
“The drug was less effective," she said, “and abortionists then had to also perform a surgical abortion, which meant that women were being charged for both chemical and surgical procedures.”
Mifepristone, which blocks the pregnancy hormone progesterone, constitutes one dose of the abortion bill. Patients have the option to reverse the effects of mifepristone before taking misoprostol, but once they take misoprostol, which induces contractions and a miscarriage, the effects are irreversible.
While pro-abortion lawmakers maintain that abortion pills are safe, pro-life groups have repeatedly warned that abortion pills have adverse side effects. Students for Life of America partnered with Charlotte Pence Bond, daughter of former Vice President Mike Pence, to create a new docuseries titled “This is Chemical Abortion,” designed to put a spotlight on the dangers of chemical abortions.
Last year, the pro-life group Live Action put together an investigative report titled, “Abortion Pill Kills,” which highlighted complications that women can experience after taking abortion pills.
These side effects include “severe cramping, contractions, and heavy bleeding.” According to the report, the bleeding can often last from nine to 16 days, but in 8% of cases, the bleeding continues for more than 30 days.
The debate about the abortion pill and the safety protocols in place regulating its administration has accelerated over the past year. As Democrats and pro-abortion activists pushed for loosening the REMS, Republican lawmakers wrote a letter to the FDA, asking the government agency to label the abortion pill as “deadly” and classify it as a “threat to women’s health.”
While a federal judge struck down the REMS last summer, enabling women to obtain the abortion pill by mail, the U.S. Supreme Court reinstated it about a week before the Trump administration left office in January. In response to the Democrats’ push to ensure easy access to abortion drugs, several states have passed laws imposing restrictions on the availability of chemical abortion.
Ryan Foley is a reporter for The Christian Post. He can be reached at: ryan.foley@christianpost.com